In this article, we’ll talk about the working pressures of refrigerant gases R404A and R290 (propane), widely used in commercial systems.
Refrigeration cycle in commercial systems
In a commercial refrigeration system, the refrigeration circuit is similar to domestic ones, but with larger dimensions and cooling capacity.
Check out the cycle that the refrigerant will complete within the circuit:
As it passes through each of these components, the fluid’s temperature and pressure changes, this is characterized by evaporating at low temperatures and condensing at high pressures (high temperatures).
With these changes, the refrigerant removes heat from within the refrigeration system (evaporator) and releases it to the external environment (condenser), thus completing the refrigeration cycle.
R290 and R404A fluid evaporating temperature and low pressure
As we saw above, the pressure and temperature variation inside a refrigeration system allows the fluid to change its physical state from liquid to gas in the evaporator and from gas to liquid in the condenser. These changes ensure heat removal from the inside to the outside of the system and thus generating refrigeration.
In the table below, you can see the difference in temperature and pressure between applications:

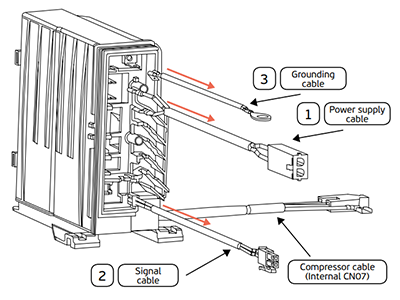
Besides this, each refrigerant has specific working pressures. When carrying out a gas charge in these systems (click here to learn how), the contractor must be attentive to the application they’re working on and the specific working pressures of the fluid. Note, for example, that the R404A gas works with an evaporator pressure greater than R290.
[box side=”alignleft” color=”box-verde” pos=”horizontal”]
Why is R290 (propane) the refrigerant which replaced R404A in commercial applications?
The first reason is linked to the thermodynamic and physical characteristics of the two refrigerants. In the compression process, R290 reaches an efficiency level of up to 60% higher than other HFCs. This means that the compressor becomes more energy efficient.
Furthermore, the R404A refrigerant is synthetic and does not easily decompose in the environment. While R290 is a natural refrigerant. It has no potential to harm the ozone layer and has negligible global warming potential. Thus, this fluid is suited to the new market requirements and North-American and European regulations.
From a technical point of view, R290 meets the ideal physical and chemical characteristics for a refrigerant, which are: low toxicity levels, chemical compatibility and stability with oil and the other components of the compressor as well as suitable operating pressures depending on the condensation and evaporation temperatures.
Its use is limited due to quantity. For safety reasons, in a refrigeration system the maximum charge of R290 is 150g. For systems that require greater fluid charge or with more than one sealed unit per system, R404A is used.
[/box]